 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
![]()
 |
 10-06-2002, 02:58 PM
10-06-2002, 02:58 PM
|
#1 |
|
John Locke
 Join Date: February 7, 2002
Location: Edmonton, Canada
Age: 35
Posts: 8,985
|
I need some help with some homework. Where can I find a periodic table that, somewhere, shows the atomic structure of the Elements? Thanks!
|

|
 10-06-2002, 03:13 PM
10-06-2002, 03:13 PM
|
#2 |
|
Drizzt Do'Urden
 Join Date: August 16, 2002
Location: Newcastle, England
Age: 45
Posts: 699
|
__________________
|

|
 10-06-2002, 03:19 PM
10-06-2002, 03:19 PM
|
#3 |
|
John Locke
 Join Date: February 7, 2002
Location: Edmonton, Canada
Age: 35
Posts: 8,985
|
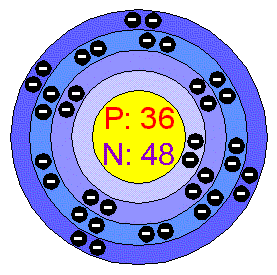
No...it needs to have a picture of the atomic structure for the elements that I can draw. Much like this, but this is completely wrong. (My teacher said so)
|

|
 10-06-2002, 05:32 PM
10-06-2002, 05:32 PM
|
#4 |
|
Apophis
 Join Date: July 10, 2001
Location: By a big blue lake, Canada
Age: 49
Posts: 4,628
|
Yes that is wrong. Letīs see if I can help you out some. You know the different designations of the electron configuration right? (K, L, M, N). K can only contain two atoms and fill up first. L can contain 8 electrons. After that the difficulty starts. Youīre not studying quantum mechanics are you? Iīll just skip the theory for that lol [img]smile.gif[/img] . M can actually contain 18 electrons. BUT first it contains 8, then N gets 2 electrons. After that you can start to fill M again up to 18 BEFORE you add to N (this is called the aufbau model. So your example would be
[ 10-06-2002, 05:35 PM: Message edited by: WillowIX ]
__________________
Confuzzled by nature. |

|
 10-06-2002, 05:36 PM
10-06-2002, 05:36 PM
|
#5 |
|
John Locke
 Join Date: February 7, 2002
Location: Edmonton, Canada
Age: 35
Posts: 8,985
|
We just call the diff things levels, but ok...So it goes 2, 8, 18, 8?
EDIT- I'm only in Grade 9! [img]tongue.gif[/img] [ 10-06-2002, 05:37 PM: Message edited by: Sir Goulum ] |

|
 10-06-2002, 05:42 PM
10-06-2002, 05:42 PM
|
#6 |
|
Jack Burton
Join Date: November 10, 2001
Location: Bathurst & Orange, in constant flux
Age: 37
Posts: 5,452
|
It does have Chemical Information
 Click the element you want, and scroll down to "Chemical Data", click HTML. Under Physical Information, it gives you what you need to know: Atomic Number 6 Relative Atomic Mass (12C=12.000) 12.011 That is from Carbon. The atomic number relates to the number of electrons and protons both, the Relative Atomic Mass is the Number of Protons plus the number of Neutrons. That gives you enough information to draw the nucleus, in this case there are 6 protons and 6.011 Neutrons, although 6 Neutrons would generally be accepted at the level you`re doing. [img]smile.gif[/img] The only complication is the electron shells: just remember the pattern 2,8,8,16 ; that should be as far as you need go [img]smile.gif[/img] EDIT: Ok, to quote from my science text book: The total number of electrons allowed in a shell n and all those before it, is 2n2. Thus, Shell Number (n) ..... Maximum Number of Electrons (2n2). 1 ..... 2 2 ..... 8 3 ..... 18 4 ..... 32 etc etc Here the book starts getting very... wierd. It gives a diagram of a Chlorine atom, with 2,8,7. But from the table it gives, it should be 2,6,8 ... I have always learned 2,8,8,16; so er... ignore 2n2  [ 10-06-2002, 05:54 PM: Message edited by: LennonCook ] |

|
 10-06-2002, 05:48 PM
10-06-2002, 05:48 PM
|
#7 |
|
Harper
 Join Date: October 2, 2001
Location: Aberdeen, Scotland
Age: 42
Posts: 4,774
|
Willow has it right. It does get even more complex in a couple years though.
Each electron shell consists of electron orbitals, each electron orbital contains up to two electrons. 1 "p" type, 3 "d" types and 5 "f" types if memory serves. Which is probably dosent, and theres one after "f" which totaly escapes me. The designation isnt important anyway, the principle is. Now, you know that shells have different energy levels, right? Well, the orbitals within those shells also have slightly varying energy levels, and in the higher shells p type orbitals have less energy than the f types from the previous level. Since its, strictly speaking, orbitals and not shells that fill up in order of energy level, the "p" type in shell "M" fills up before the "f" types in shell "N" Now are you glad that you dont have to study the theory behind all this for another couple years? Anyway, I usualy find it easier to remember stuff like this by learning the reason why, so maybe some of this will be useful.
__________________
[img]\"http://www.sighost.us/members/Zvijer/andrewas.gif\" alt=\" - \" /> |

|
 10-06-2002, 05:49 PM
10-06-2002, 05:49 PM
|
#8 | |
|
John Locke
 Join Date: February 7, 2002
Location: Edmonton, Canada
Age: 35
Posts: 8,985
|
Quote:
|
|

|
 10-06-2002, 05:50 PM
10-06-2002, 05:50 PM
|
#9 | |
|
Apophis
 Join Date: July 10, 2001
Location: By a big blue lake, Canada
Age: 49
Posts: 4,628
|
Yes Sir G that would be correct (not your post right above this one, but further up [img]smile.gif[/img] ). I found this for you, it has the number of electrons in ech "level" listed [img]smile.gif[/img] http://www.chemicalelements.com/show...onconfig.html.
Quote:
[ 10-06-2002, 05:51 PM: Message edited by: WillowIX ]
__________________
Confuzzled by nature. |
|

|
 10-06-2002, 05:51 PM
10-06-2002, 05:51 PM
|
#10 | |
|
Harper
 Join Date: October 2, 2001
Location: Aberdeen, Scotland
Age: 42
Posts: 4,774
|
Quote:
Actualy, IIRC, N can hold up to 32, but thats getting may beyond anything I officialy studied.
__________________
[img]\"http://www.sighost.us/members/Zvijer/andrewas.gif\" alt=\" - \" /> |
|

|
| Currently Active Users Viewing This Thread: 1 (0 members and 1 guests) | |
|
|
 Similar Threads
Similar Threads
|
||||
| Thread | Thread Starter | Forum | Replies | Last Post |
| Anyone willing to help me a bit?(Not homework) | Neb | General Conversation Archives (11/2000 - 01/2005) | 4 | 11-21-2002 11:42 AM |
| Homework | Neb | General Conversation Archives (11/2000 - 01/2005) | 24 | 09-17-2002 07:23 AM |
| Woo! Homework help! | Lady Blue03 | General Conversation Archives (11/2000 - 01/2005) | 13 | 09-05-2002 09:08 AM |
| Homework Help... | Deathmage | General Conversation Archives (11/2000 - 01/2005) | 5 | 08-28-2002 12:52 AM |
| Homework Help | Deathmage | General Conversation Archives (11/2000 - 01/2005) | 4 | 05-08-2002 08:32 AM |